[Light] [Air] [Water] [Grow Matrix / Medium] [Nutrients]
https://www.apogeeinstruments.com/PAR METER TOOLS
energy meter able to measure PPFD at a certain moment, or capture a series of measurements with a time interval.
Introduction:
To absorb light, you need to have things work just right.
You may have heard that light is quantized, what this means is that it only gets absorbed in specific chunks, one photon at a time. And all the energy of that photon has to go somewhere.
It turns out there are a few different places for that energy to go, and since each color of light has different energy, those different absorption mechanisms affect the colors differently.
Ultraviolet has the highest energy, it’s absorbed into the electrons in a material, kicking them up in energy or ejecting them from the atoms entirely.
Infrared light is absorbed into the vibrations of the atoms and molecules in a material.
Different materials have different thresholds for these absorption methods, and a huge difference is whether things are metals or not.
Metals have completely different architectures for their electrons, but the basic concepts of “need to absorb a whole photon” still apply.
For glass, visible light isn’t high enough energy to be absorbed by the electrons and too high to be absorbed as a vibration.
Remember, it’s all or nothing - you can’t absorb half a photon.
It gets a bit more complicated since you also have to absorb the momentum of the photon, and not matching the quantized momentum kick will lead to the photon not getting absorbed either.
:::: About LIGHT! ::::
Light visible to humans is only a small part of the Electromagnetic Spectrum, which also contains microwaves, radio waves, and radiation.
Visible light is an electromagnetic wave, consisting of oscillating electric and magnetic fields traveling through space. Other light frequencies are invisible to our eyes, such as Ultraviolet (UV-A,B,C) and Infrared.
Nanometer (nm) measurement of wavelength (λ) are used to describe common grow light frequencies.
In physics, the wavelength is the spatial period of a periodic wave—the distance over which the wave's shape repeats.
Visible light is usually defined as:
Having wavelengths in the range of 400–700 nanometers λ (nm) between the infrared (with longer wavelengths) and the ultraviolet (with shorter wavelengths).
This wavelength means a frequency range of roughly 430–750 terahertz (THz).
"Frequency" is an important parameter used in science and engineering to specify the rate of oscillatory and vibratory phenomena such as mechanical vibrations or audio signals (sound); radio waves; and light.
The wavelength of energy which stimulates chlorophyll in plants is what we often see as Red and Blue light.
Plants are perceived as green because chlorophyll absorbs mainly
blue and red wavelengths and reflects the green!
Anthocyanins are other plant pigments; depending on their pH, they may appear red, purple, blue or black. Anthocyanins are found in a cell vacuole, mostly in flowers and fruits, but also in leaves, stems, and roots. The absorbance pattern responsible for the red color of anthocyanins may be complementary to that of green chlorophyll.
Betalains are a class of red and yellow tyrosine-derived pigments found in plants of the Caryophyllales (including the cacti, carnations, amaranths, ice plants, beets, and many carnivorous plants), where they replace anthocyanin pigments. The particular shades of red to purple are distinctive and unlike that of anthocyanin pigments found in most plants. ++++
https://www.horti-growlight.com/knowledge
"Short day plants" change from the vegetative growth stage to the floral growth stage when they are exposed to critical short day lengths.
For cannabis this is 12 hours of light to trigger flowering. Most growers keep cannabis to 18 hours of light during the vegetative growth stage until time to flower, unless plant is an Auto-Flower -- which flowers after a certain length of time (~90 growing days), not due to a change in photo period!
++UV LIGHT++
UV‐B RADIATION EFFECTS ON PHOTOSYNTHESIS, GROWTH
and CANNABINOID PRODUCTION OF TWO Cannabis sativa CHEMOTYPES
Does UV Light Increase Cannabis Potency?
Is UV Light Important for Cannabis?
UVB Light And Boosting THC Potency
++++++++++
UV Wavelengths and Spectral Sensitivity of Disinfection
The UV spectral region ranges from 100 nm to 400 nm and is usually divided into three sub-regions based on absorption in the atmosphere and the biological action of radiation:
UVA: 315 nm to 400 nm
UVB: 280 nm to 315 nm
UVC: 200 nm to 280 nm
UVA and UVB are transmitted through the earth’s atmosphere and have limited germicidal effects.
On the other hand, UVC is completely absorbed by the earth’s atmosphere and is highly disruptive for live organisms because it is strongly absorbed by proteins (principally 210 nm to 230 nm) and the nucleic acids of DNA and RNA (principally 250 nm to 280 nm).
The latter wavelength range is commonly referred to as the “germicidal UVC range.”
The spectral sensitivity of a microbe is the relative ability of the microbe to absorb a photon as a function of wavelength over a range of wavelengths.
Pathogens have a unique radiation absorption “fingerprint,” which means that they absorb photons differently at varying wavelengths.
While different, each pathogen shows a peak absorption near 265 nm and diminishes rapidly above 280 nm in the UVB range.
For the most pathogens, there is a steep drop in sensitivity below 250 nm.
Comparing the Impact of Far UVC and Germicidal UVC on Disinfection
Within the germicidal UVC range, 260 nm to 270 nm is seen as an ideal wavelength, with only a small drop in efficacy in damage to the nucleic acid across that wavelength range (peak DNA/RNA absorption is observed between 263 nm to 265 nm) while, outside that range, the efficacy of longer or shorter wavelengths starts to fall drastically.
For comparison, UVC in that range is two to three orders of magnitude more effective than UVA at inducing DNA damage.
The primary process of disinfection in the germicidal range is by the generation of cyclobutane pyrimidine dimers (CPD), the dominant form of UV-induced genomic damage.
These dimers interrupt the replication of DNA/RNA and lead to bacterial cell death and viral inactivation.
Recently, scientists have studied the application of Krypton-Bromine and Krypton-Chlorine excimer lamps to generate primary photon emission peaks at 207 nm and 222 nm, respectively.
UVC in the 207 nm to 222 nm range is commonly referred to as Far UVC. While photons emitted in this range are absorbed to some degree by the nucleic acids of DNA/RNA, the principal factor in reducing infectivity is thought to result from absorption and resultant damage to proteins. This has been demonstrated notably on adenovirus, methicillin-resistant staphylococcus aureus (MRSA), and influenza virus of type H1N1.
In water applications, the use of 222 nm does not seem likely since the UV transmissivity (UVT) in water becomes unacceptably large.
UVT for filtered water is approximately constant down to 260 nm and starts to drop dramatically at shorter wavelengths due to common chemical contaminates such as nitrates.
In addition, the pathogens of interest are biofilm-forming bacteria such as pseudomonas with peak absorption between 260 nm to 265 nm which exhibit lower photon absorption at shorter wavelengths.
Employing a 205 nm to 230 nm photon source to treat pathogens is far more likely to depend on the protein aspect of a pathogen, which can have substantially different absorption coefficients, rather than the proven nucleic acid DNA/RNA approach utilizing the absorption peak in the 260 nm to 270 nm wavelength range which has been shown to consistently and predictably inactivate pathogens.
======
!!Always perform your own case-specific economic analysis
to help decide what lamp type is the best investment.!!
---Light and Plants --
How much GROW light spectrum do they need, and what do our lights produce??
 |
| Figure 1. Two lamps being tested in a specialized room at Utah State University. (Photos: Jakob Johnson, Utah State University) |
The total output of a lamp can be measured by either flat plane integration (Figure 1) or by an integrating sphere, which is a hollow sphere that is covered with a white, highly reflective coating inside to reflect light. A lamp is inserted, and light output and the electrical input (including any ballast) are measured. An integrating sphere is expensive to purchase and requires expertise to operate and thus, these measurements are regularly done by independent lighting laboratories and lighting companies.
++++++++++++++
Lumens measure light intensity. One lumen is equal to one candela. Lumens only relate to human eye sensitivity – they don’t tell you anything about plant grow lights. Lumens are great for how well a lamp will let you see in the dark, but that’s it. "Luminous flux" differs from power ("radiant flux").
"Radiant flux" includes all electromagnetic waves emitted, while "luminous flux" is weighted according to a model (a "luminosity function") of the human eye's sensitivity to various wavelengths; One "lux" is one lumen per square meter.
Lumens (below, in relation to HPS/Metal halide bulb outputs) is associated with human vision;
PAR (Photo-synthetically Active Radiation, above) is for plant chlorophyll
+++++
Photosynthetic Photon Efficacy (PPE).
PPE is the
PAR photon output (unit of micromoles per second, or μmol·s–¹)
divided by the
input power (watts, or W) to produce that light. The unit becomes μmol·s–¹·W–¹, and because one watt (W) equals one joule per second (J·s–¹), the ratio can be simplified to μmol·J–¹
(μmol per second/joule per second) -- or,
PPE. That means that for every joule of electrical energy (joule = watt * second) a certain number of "photon micromoles" are produced per second. 1 micromole is 602,000,000,000,000,000
photons.
A 400-W single-ended high-pressure sodium lamp (HPS) with a magnetic ballast has a PPE value of approximately 0.9 μmol·J–¹ while a double-ended 1,000-W HPS lamp with an electronic ballast has a PPE of around 1.7 μmol·J–¹.
The PPE value for LED products ranges considerably; many new fixtures exceed 2.0 μmol·J–¹.
The higher PPE value, the more effective it is at converting electricity into photosynthetic photons. The PPE of LEDs continues to increase and purchase costs are decreasing, so some growers are hesitant to invest. The theoretical maximum PPE for LEDs is 4.6 to 5.1 μmol·J–¹, depending on the composition of the LEDs used in an array. We are unlikely to achieve these values in our lifetimes, but an efficacy of 3.5 μmol·J–¹ is possible in the next decade. (~2027)
+++
Photosynthetic Photon Flux (PPF): Measures total amount of light produced by a grow light in terms of micromoles of photons produced per second (often written as umol/s or μmol/s). This tells you the full amount of light coming from the LED grow light.
 |
| GE Grow Light Bulb, PAR38 Grow Light Bulb for Indoor Plants, Full Spectrum, 32-Watt, Balanced Lighting for Seeds and Greens - high output PPF of 50 micromoles per second. |
| |
Photosynthetic Photon Flux Density (PPFD):Measures the amount of micromoles of photons striking a square meter per second (often written as umol/m2/s, μmol/m2/s, or μmolm-2s-1).
Because the Sun’s intensity is only that bright for a small portion of the day and because the angle of that intensity changes throughout the day, providing that much light for an extended period of time would very likely be damaging to your plant. Intense light has high PPFD and low-intensity light has low PPFD.
A ‘light response curve’ shows how effectively a plant utilizes light at differing intensities.
Depending on the plant, at levels greater than 800-1000 μmol/m2/s the efficiency that a plant uses the light starts to slow. Meaning, you can provide your plant more light than this, but you might not see a huge change in outcome.
For instance, a lettuce plant needs ~80 µmol/m2/s during seedling, ~150 µmol/m2/s during vegetative phase and above 200 µmol/m2/s when it starts to flower.
Recommended PPFD levels for the establishment phase of cannabis are 75-150 µmol/m²/s for cuttings, and 100-300 µmol/m²/s for seedlings.
For cannabis in the vegetative stage, PPFD levels of 300-600 µmol/m²/s are recommended and may be increased to over 600 µmol/m²/s in the reproductive phase.
Full daylight sun at noon in the summer is around 2000 μmol/m2/s. During the evening, before sundown, it's down to 500-1000. During dark nights, close to zero.
Some plants thrive in warm climates while others do better in cold and shaded places. This means that some plants prefer intense sunlight (high PPFD) while others prefer less intense (low PPFD). Same goes for our everyday vegetables, herbs, fruits, houseplants, and flowers.
Time is also a factor. Low intense light but over a long period results in the same number of photons as twice the light intensity but only during half as long period of time. This is especially important to indoor grows where the light output of a grow light and its photoperiod (how long the light is on) can easily be regulated.
Very high PPFD levels over a short duration of time or very low PPFD levels over a long duration of time are rarely ideal for good growth.
Understanding PPFD and DLI (see below) is essential to successfully setup an efficient grow.
++
Daily light integral (DLI) describes the number of photosynthetically active photons (individual particles of light in the 400-700 nm range) that are delivered to a specific area over a 24-hour period.
This variable is particularly useful to describe the light environment of plants.
In other words:
DLI describes the sum of the per second PPFD measurements during a 24-hour period.
DLI is usually calculated by measuring the photosynthetic photon flux density (PPFD) in μmol·m−2·s−1 (number of photons in the PAR range received in a square meter per second) as it changes throughout the day, and then using that to calculate total estimated number of photons in the PAR range received over a 24-hour period for a specific area.
The formula for calculating DLI is:
μmol m-2s-1 (or PPFD) x (3600 x photoperiod) / 1,000,000 = DLI (or moles/m2/day)
 |
| The number of moles per hour, per m2, multiplied with photoperiod (the number of hours with that intensity) = DLI, Daily Light Integral |
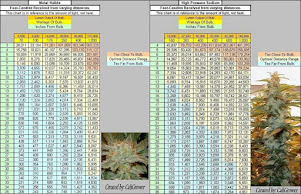
Calculation Example:
You are growing cannabis and want to give them a range from 22-30 DLI, and choose 25 DLI.
Your grow light produces 500 PPFD at 18" from the plant according to your grow light PPFD chart/light footprint.
If the lamp's distance is increased to 20" from the plant, it produces 400 PPFD at the canopy.
If the lamp is lowered to 15", it's producing 700 PPFD.
By looking at the above chart we see that ~25 DLI is achieved by exposing the plant to:
- 500 PPFD over 14 hours but also when exposing the plant to
- 400 PPFD over 17 hours or
- 700 PPFD over 10 hours.
Choose a combination that fits both your grow set up but also your plant's minimum and maximum PPFD limits.
18 hrs at 450 ppfd (umols/meter sq/second) (grow), 12 hours at 700 ppfd (bloom)
as a light intensity "average" seem to be ideal for cannabis grow cycles.
Distance of lights to plants will change the light per meters squared number, and adjust it as needed.
This is why lights are higher during veg, and lower during bloom because of photoperiod changes from 18 to 12 hours; total power can be less consumptive in veg.
Putting aside the limitations of scientific results (Links to Chandra Cannabis growth studies below), reaping an average intensity of 2,000 µmol/m2/s with natural light isn't possible. And, although maintaining 2,000 µmol/m2/s over a typical 18 hour light cycle is possible with artificial light, it may not be cost-effective. So, what's a realistic light intensity that's less than 2,000 µmol/m2/s? It seems that a practical maximum has already been determined. When Chandra studies Cannabis, he maintains a crop of test plants and relies on 700 µmol/m2/s in the following setup:
Area: 335 to 350 square feet.
Lights: 14 1,000 watt HID's with cooling fans attached to each.
Light height: 3 to 4 feet from the canopy.
PPFD: 700 µmol/m2/s (+/-24 µmol/m2/s) at the canopy.
Light cycles: 18/6 and 12/12.
Temperature: 25 C (+/- 3 C).
Humidity: 55 % (+/-5 %).
Containers: 30 cm diameter x 28 cm high.
Growth medium: 1:1:1 of topsoil, sand and manure.
Ambient CO2 is assumed, because it's not mentioned.
Although the above doesn't probe the limits of Cannabis growth, I assume Chandra utilizes this setup, because it's an effective use of resources that produces a reasonable crop. So, if you replicate this environment and target 700 µmol/meter2/second as a light intensity average, your grow has as much potential as Chandra's.
Regardless of numbers, it is essential to look for signs that plants are receiving too much light. As plants are exposed to an excess of light, they take in more nutrients than they otherwise would, leading to a buildup of nutrients in the plant and causing a condition known as nutrient burn.
It’s worth noting that some LED companies can increase their PPFD numbers by measuring extremely close to the grow light or using spot-light like reflectors or lenses. An LED company should always report what distance their PPFD numbers were taken at (e.g., 24 in, etc.).
++
Another measurement relevant to DIY LED grow light enthusiasts is wall plug efficiency (WPE). This is a ratio of the amount of energy put in and the amount of light produced. This can be expressed as a percentage, such as 60% “wall plug efficiency,” which means that 60% of the electricity that goes through the light gets converted into light. The rest gets turned into heat that will need to be dealt with in the grow light itself as well as the room that houses the light.
It’s not typical to rate a grow light’s wall plug efficiency, but high-quality diodes made specifically for horticulture occasionally have this listed. For instance, high quality blue LEDs at 450nm can reach wall plug efficiencies of 60%, red LEDs at 660nm with 50% WPE, and green 530nm with 25% WPE. Wall plug efficiency can be calculated using a diode’s radiant flux (not luminous flux, which is a measure of how bright a light appears to the human eye and not how many photons it is producing) divided by the total wattage of electricity the diode uses. Remember to convert between milliwatt and watt, as necessary (1000 milliwatts = 1 watt).
++INFRARED "LIGHT" ENERGY++
The protein
Phytochrome is the only known receptor that is sensitive to far-red/infrared wavelengths.
Phytochromes are a class of
photoreceptor in plants, bacteria and fungi, sensitive to light in the red and far-red region of the visible spectrum and can be classed as either Type I, which are activated by far-red light, or Type II that are activated by red light.
Phytochromes control many aspects of plant development. They regulate the germination of seeds (photoblasty), the synthesis of chlorophyll, the elongation of seedlings, the size, shape and number and movement of leaves and the timing of flowering in adult plants.
Research has shown combining blue light and red light with far-red/infrared light (700-760nm) led to an increased rate of photosynthesis due to the
Emerson effect.
The Emerson effect is the increase in the rate of photosynthesis after chloroplasts are exposed to light of wavelength 680 nm (deep red spectrum) and more than 680 nm (far red spectrum). The effect was early evidence that two photosystems, processing different wavelengths, cooperate in photosynthesis.
Too much IR radiation can also be an issue because to plant the majority of IR radiation is felt as heat. Growers who use traditional 1000W HPS lighting, which produces excess heat through IR radiation, have to install and operate expensive HVAC systems to mitigate the heat.
Too much IR radiation can cause plants to stretch spreading out the plant nodes, can discolor the leaves or even kill the plants.
Choosing a light with the right appropriate amount of far-red and infrared light is key for healthy and natural plant growth.
One of the most overlooked but most important specification of IR illuminators is frequency. Most IR illuminators on the market are not one-hundred percent invisible to the human eye. Illuminators operating in the ranges of 750nM and 840/880nM emit a soft red glow when looking directly at the illuminator itself. For true stealth operation, select an illuminator that works at 940nM or greater.
IR coming from the camera are not strong enough coming from the camera to affect any change in the plants. The camera isn't putting out as much energy as the sun. IR can't be seen by the human eye, but is felt as heat because it reacts with molecules. So something very strong would affect the plant, literally cooking it from the inside out.
======
*The einstein is a uncommon unit defined as the energy in one mole of photons (6.022×1023 photons).
Because energy is inversely proportional to wavelength, the unit is frequency dependent. This unit is not part of the International System of Units and is redundant with the joule. In studies of photosynthesis the einstein is sometimes used with a different definition of one mole of photons. As such, photosynthetically active radiation (PAR) was formerly often reported in microeinsteins per second per square meter (μE m−2 s−1). This usage is also not part of the International System of Units and when used this way it is redundant with the mole.










No comments:
Post a Comment